Book Appointment Now

Alligation Math Practice Problems for PTCB Exam
Mastering alligation math is a crucial skill for anyone preparing for the Pharmacy Technician Certification Board (PTCB) exam. This method, used to calculate the correct proportions of different concentrations to achieve a desired mixture, is frequently tested.
Practice Problems
Alligation Medial Problems
- Problem 1: You have two solutions: Solution A with a concentration of 20% and Solution B with a concentration of 50%. How much of each solution is needed to prepare 300 mL of a 30% solution?
- Problem 2: A pharmacist needs to prepare 500 mL of a 25% alcohol solution. They have a 10% alcohol solution and a 60% alcohol solution available. How much of each solution should be used?
- Problem 3: You need to prepare 400 mL of a 35% solution. You have Solution C with a concentration of 25% and Solution D with a concentration of 70%. Determine the volume of each solution required.
Each problem requires you to use the alligation medial method to find the correct proportions of each solution to achieve the desired final concentration. Try solving these independently before checking the solutions provided in the next section.
Solutions to Practice Problems
Alligation Medial Solutions
Solution to Problem 1: You have two solutions: Solution A with a concentration of 20% and Solution B with a concentration of 50%. How much of each solution is needed to prepare 300 mL of a 30% solution?
Step-by-Step Solution:
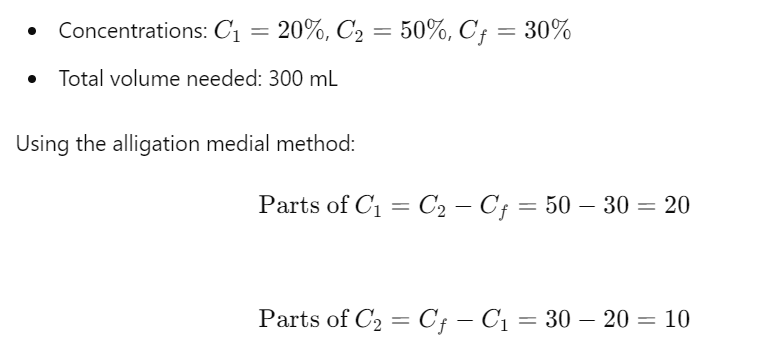
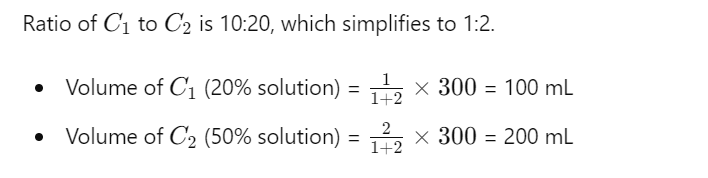
So, you need 100 mL of Solution A and 200 mL of Solution B.
Solution to Problem 2:
A pharmacist needs to prepare 500 mL of a 25% alcohol solution. They have a 10% alcohol solution and a 60% alcohol solution available. How much of each solution should be used?
Step-by-Step Solution:
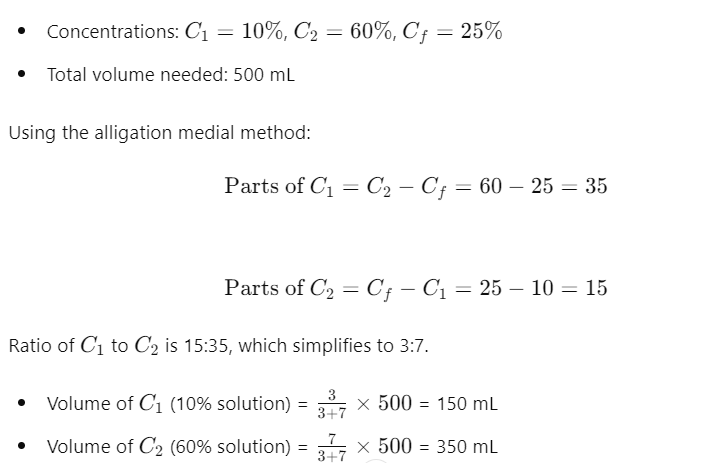
So, you need 150 mL of the 10% solution and 350 mL of the 60% solution.
Solution to Problem 3: You need to prepare 400 mL of a 35% solution. Solution C has a concentration of 25%, and Solution D has a concentration of 70%. Determine the volume of each solution required.
Step-by-Step Solution:
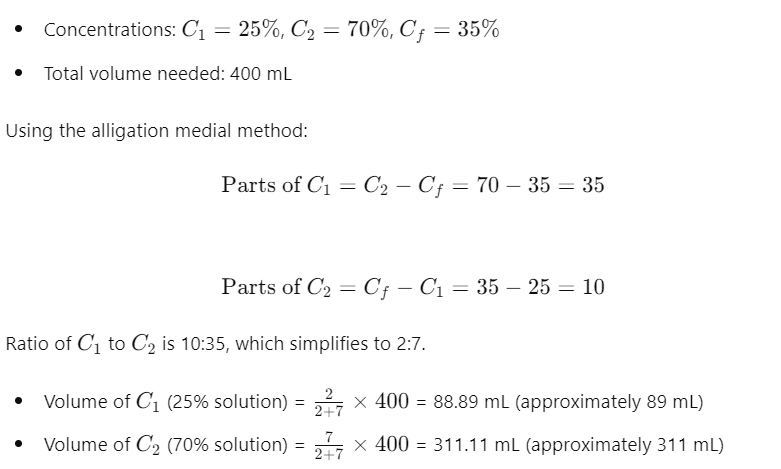
So, you need 89 mL of the 25% solution and 311 mL of the 70% solution.
Quick Guide to Alligation
What is Alligation?
Alligation is a mathematical method used to calculate the proportions of different solutions needed to achieve a desired concentration. It is particularly useful in pharmacy for mixing solutions of different strengths to obtain a specific concentration required for a prescription.
Practical Applications in Pharmacy:
- Compounding medications to achieve precise drug concentrations
- Adjusting the strength of a solution by mixing different concentrations
- Diluting or fortifying solutions for patient-specific needs
Alligation Medial
Alligation Medial is used when you need to find the average concentration of a mixture created from two or more solutions with known concentrations. It determines the overall concentration of the resulting mixture.
Basic Example: Suppose you mix 100 mL of a 20% solution with 200 mL of a 50% solution. To find the concentration of the resulting mixture:
- Multiply the volume of each solution by its concentration:
- 100 mL × 20% = 2000
- 200 mL × 50% = 10000
- Add these values: 2000 + 10000 = 12000
- Divide by the total volume of the mixture:
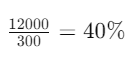
The resulting concentration is 40%.
Alligation Alternate
Alligation Alternate is used to determine the ratio in which two solutions of different concentrations should be mixed to achieve a desired concentration. It involves a simple mathematical approach to solve for the required volumes.
Basic Example: To prepare 300 mL of a 30% solution using a 20% solution and a 50% solution:
- Subtract the desired concentration from each of the given concentrations:
- 50% (higher) – 30% (desired) = 20 parts
- 30% (desired) – 20% (lower) = 10 parts
- The ratio of the two solutions is 10 parts of the 50% solution to 20 parts of the 20% solution, which simplifies to 1:2.
- To prepare 300 mL, use:

This method provides a straightforward way to determine the required volumes to achieve the desired concentration.
Tips for Success
Common Mistakes to Avoid
- Incorrectly Setting Up the Problem:
- Always double-check that you have correctly identified the higher and lower concentration solutions and the desired concentration.
- Ensure you are clear on the total volume needed and the units being used.
- Misinterpreting the Alligation Grid:
- When using the alligation grid method, make sure to properly set up the parts of each concentration correctly. Confusing the differences can lead to incorrect ratios.
- Math Errors:
- Simple arithmetic errors can throw off your calculations. Recheck your subtraction and division steps.
- Make sure to simplify ratios correctly and apply them accurately to the total volume.
- Ignoring Practical Considerations:
- Consider the solubility and compatibility of the solutions being mixed in real-life applications.
- Verify that the resulting mixture’s concentration is realistic and achievable.
Quick Tips for Solving Alligation Problems Efficiently
- Understand the Concepts:
- Make sure you have a solid grasp of the concepts of concentration and volume.
- Familiarize yourself with both Alligation Medial and Alligation Alternate methods.
- Use the Alligation Grid:
- Set up an alligation grid to visually map out the differences between concentrations. This can help you avoid mistakes and simplify the calculation process.
- Practice Regularly:
- Consistent practice with different types of problems will help reinforce your understanding and improve your speed and accuracy.
- Break Down the Steps:
- Break complex problems down into smaller, manageable steps. This makes them easier to follow and reduces the likelihood of errors.
- Check Your Work:
- After solving the problem, review your steps and calculations to ensure everything is correct.
- If time permits, try solving the problem using a different method to verify your answer.
Additional Resources
Recommended Textbooks and Online Resources:
- Textbooks:
- “Pharmacy Calculations for Pharmacy Technicians: A Worktext“ by Jahangir Moini
- “Pharmaceutical Calculations“ by Howard C. Ansel
Conclusion
Mastering alligation math is essential for success in the PTCB exam and for practical pharmacy applications. By understanding how to mix different concentrations accurately, you can ensure the correct dosage and efficacy of medications. The practice problems and solutions provided here are designed to help you build confidence and proficiency in this crucial area.
Keep practicing these problems and make use of the additional resources to further enhance your skills. Consistent practice will prepare you for the exam and improve your accuracy and efficiency in real-world pharmacy settings.
If you found this post helpful, please share it with others preparing for the PTCB exam or needing a refresher on alligation math. We would love to hear your thoughts and any questions you might have in the comments section below.
For more pharmacy-related content and updates, consider subscribing to our blog. Stay tuned for more tips, practice problems, and resources to help you succeed in your pharmacy career.

Founder of the PTCBFreePracticeTest.com.



